Choosing the right evaluation method depends on your specific needs and constraints. Heuristic evaluations offer quick, cost-effective insights, while usability studies provide in-depth user feedback. Task analysis enhances both methods by focusing on user tasks and uncovering challenges. By integrating these approaches, you can achieve a comprehensive and user-centric evaluation of your product.
When Do You Need a Usability Study vs. a Heuristic Evaluation? Where Does Task Analysis Fit In?
Understanding when to use a usability study versus a heuristic evaluation can significantly impact the effectiveness of your product development process. Here’s a breakdown of each method, how task analysis fits in, and examples of how these methods have been applied in our day-to-day work.
HEURISTIC EVALUATION
What is it? A heuristic evaluation involves usability experts assessing a design based on established “rules of thumb.” Historically used for human-computer interaction systems, this method evaluates software interfaces for usability. Building on well-established guidelines, experts have tailored 14 Usability Heuristics specifically for medical devices. We apply these 14 heuristics to conduct thorough heuristic evaluations, ensuring usability excellence in client projects.
Why Use It? Heuristic evaluations are quick and cost-effective, making them ideal when time and resources are limited. They provide valuable insights early in the product development process, even before a fully-fledged prototype is available. Our approach includes:
- Expert Reviews: Conducted by seasoned usability experts.
- Tailored Heuristics: Using our custom heuristics for medical devices.
- Comprehensive Analysis: Identifying potential areas for improvement and their impacts.
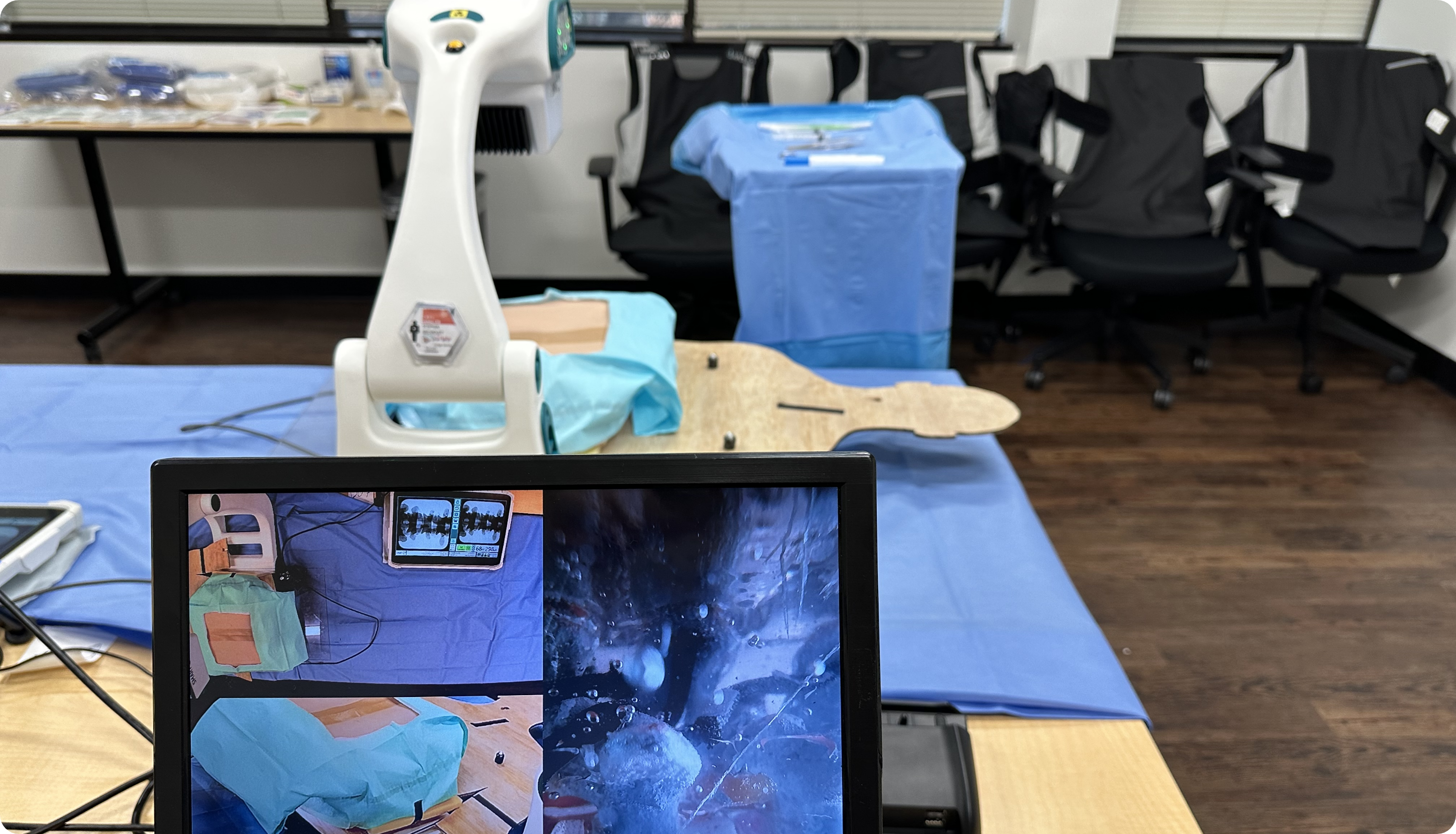
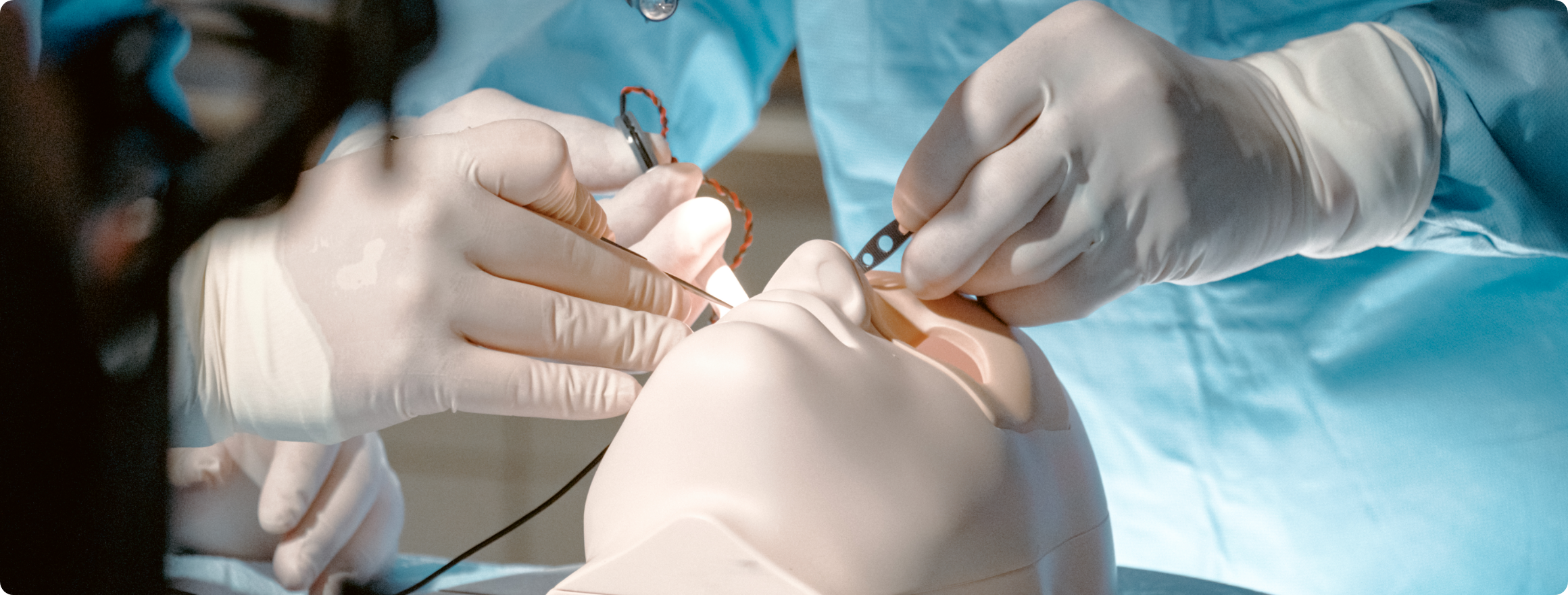
FORMATIVE USABILITY STUDY

What is it? At a high level, a usability study typically involves simulated use testing of a product to observe how users interact with it. Usability studies inform product design, identify use-related risks, and can help engineers and designers discover the root cause of use errors to include risk mitigations in the product’s design. This evaluation method uses representative users in a representative environment to gather user feedback on specific product components, or the product as a whole.
Why Use It? For medical devices and combination products, FDA requirements necessitate usability studies to ensure device safety and effectiveness. These studies help iterate through device design and thoroughly evaluate components. Compared to heuristic evaluations, usability studies show how end users interact with the device and reveal more opportunities for design improvements.
Our approach includes:
- Simulated Use Testing: Observing real users in a controlled environment.
- FDA Compliance: Ensuring all regulatory requirements are met.
- Comprehensive Study Design: Simulating a representative use environment, recruiting representative users, and applying study findings to design recommendations.
TASK ANALYSIS
What is it? Task analysis goes hand in hand with both heuristic evaluations and usability studies. It involves breaking down the tasks users will perform with the device to understand their needs and challenges.
Why Use It? It provides a deeper understanding of user interactions, enhancing the effectiveness of heuristic evaluations by ensuring that the heuristics are applied in a context that reflects real user behavior. It also enriches usability studies by identifying specific tasks that need to be tested, ensuring comprehensive coverage of user interactions. Our task analysis process includes:
- User-Centric Focus: Understanding user needs and challenges.
- Ethnographic Methods: Observing users in their natural environment.
- Detailed Task Breakdown: Analyzing each step users take with the product.
How These Methods Work Together
By combining heuristic evaluations, usability studies, and task analysis, you can ensure a thorough and user-centric design of your product. These evaluation methods complement each other, providing a comprehensive understanding of both potential usability issues and real-world user interactions.
For more detailed examples and insights, check out our case studies on heuristic evaluations and usability studies. These case studies illustrate how Kaleidoscope Innovation has successfully applied these methods to improve product design and usability.
For over 7 years, Kaleidoscope Innovation has been a trusted partner to industry leaders like Eli Lilly, Pfizer, and Baxter, helping bring safer, smarter medical products to market. Our integrated Human Factors expertise ensures that the right evaluation methods—whether heuristic, usability-focused, or task-based—are applied at the right time. Whether you're developing a new device or improving an existing one, we’re here to guide your team with insights that reduce risk, streamline development, and enhance user experience. Let’s talk about how we can help you choose and apply the best evaluation methods for your product.
Back to Insights + News




 Design and Prototyping
Design and Prototyping Formative Usability Testing and Evaluation
Formative Usability Testing and Evaluation