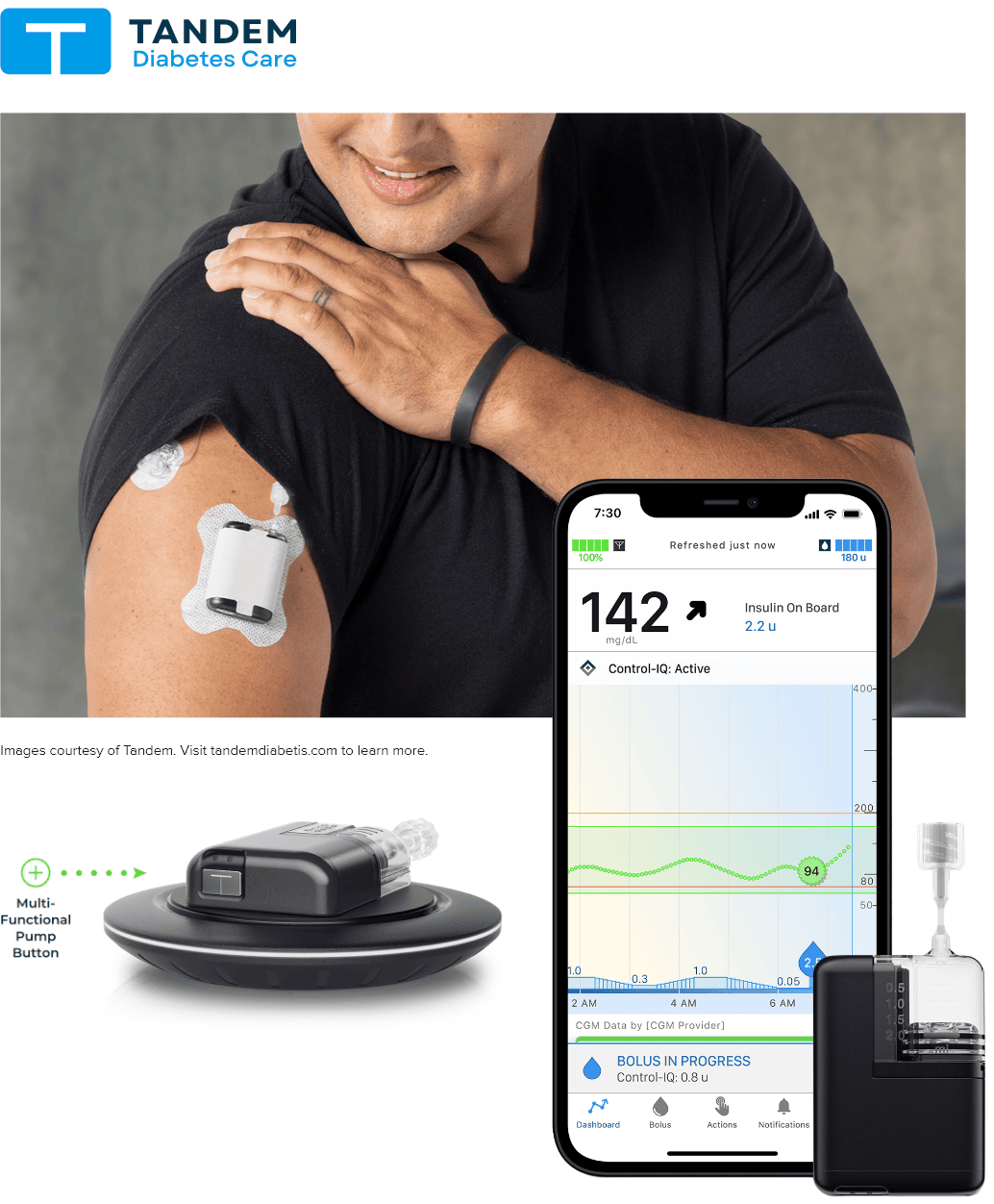
Kaleidoscope Innovation Human Factors Engineering works with clients to produce devices and experiences informed by physical and psychological research and insights. From wearable tech and surgical devices, digital health and warehouse layout and operations, our team has specialized expertise in medical devices, combination products, and consumer products.
Our human factors engineers have worked with manufacturers including Amgen, Baxter, Ethicon, and Medtronic.