In the medical product industry, ensuring the safety, efficacy, and usability of products is paramount. One of the key methods for achieving this goal is through the proper implementation of a robust Human Factors engineering (also known as usability engineering or ergonomics) process. Human Factors engineering (HFE) focuses on optimizing the interaction between people and technology to prevent errors, enhance performance, and improve the overall user experience.
Because of the risks involved, medical device development demands an especially thorough Human Factors process. They often require precise operation under conditions where an error can result in reduced clinical effectiveness, harm, or even death. Whether these products are used by health care providers, caregivers, or even the patients themselves, medical products that are not designed with the capabilities of the end user in mind can increase the chance of errors. To mitigate these risks, regulatory bodies such as the U.S. Food and Drug Administration (FDA) mandate that Human Factors principles be integrated into the design and development of medical products.
The HFE process for medical products is a structured, iterative approach that aims to identify potential use-related risks early in the design process and systematically reduce those risks throughout product development. This process incorporates both user needs and environmental considerations to create intuitive, safe, and effective products.

User Research and Task Analysis
The first step in the HFE process is to develop a deep understanding of the intended users and the tasks they will perform with the product. It is important to recognize that there may be distinct types of users (user groups) that have varying tasks, capabilities, and responsibilities. This step involves the following activities:
- User needs: Identify the specific requirements and expectations of users that the product needs to fulfill to be used safely and effectively. These needs generate testable design requirements.
- User characteristics: Understand the characteristics of the intended users that could impact product use. Common characteristics include age range, experience, education, physical abilities, cognitive capabilities, and any disabilities. These characteristics may drive segmentation of the intended users into separate user groups for usability testing.
- Use environment: Identify factors such as lighting, noise, and other products in the intended use environment that may impact use of the product, keeping in mind there may be multiple environments in which the product is used, such as a clinic or a home setting.
- Task Analysis: Based on the intended use of the product, identify and break down the tasks that users must accomplish to safely and effectively use the product. Read about Task Analysis here.
Identifying and Analyzing Risks
Once the user characteristics, environments, and tasks are understood, the next step is to conduct a use-related risk analysis (URRA). This process involves:
- identification of what and how use errors may occur
- assessment of the potential consequences and severity of those errors
- identification of potential mitigations
- validation plans for those mitigations
By systematically analyzing use-related risks, the design team can prioritize which potential issues are most critical to address based on severity, likelihood, and impact on patient safety. Additionally, this is the step in which critical tasks are identified. According to the FDA guidance document “Applying Human Factors and Usability Engineering to Medical Devices,” critical tasks are those which, if performed incorrectly or not performed at all, would or could cause serious harm to the patient or user, where harm is defined to include compromised medical care. Identification of critical tasks drives creation of the usability validation protocol, to ensure that any critical use error mitigations are properly validated.
The URRA can be considered a “living document,” since it should be continuously updated throughout the product development process when new hazards are identified through usability testing and existing hazards are mitigated through design.
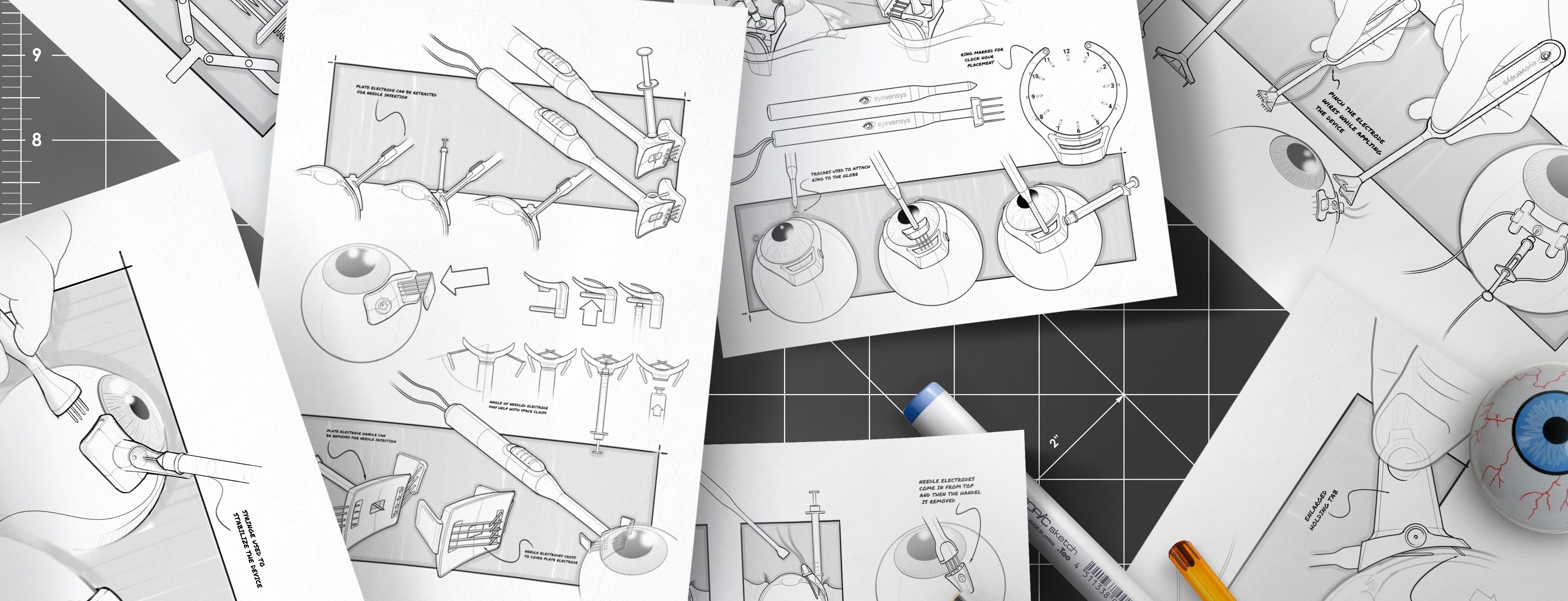
 Design and Prototyping
Design and Prototyping
Human Factors specialists collaborate closely with designers and engineers—and and at Kaleidoscope, that means working under the same roof—to create prototypes that align with user needs and risk mitigation strategies. This integrated approach streamlines communication, accelerates iteration, and results in more effective, user-centered designs. Multiple prototypes may be developed that can be evaluated with representative users in the next phase.
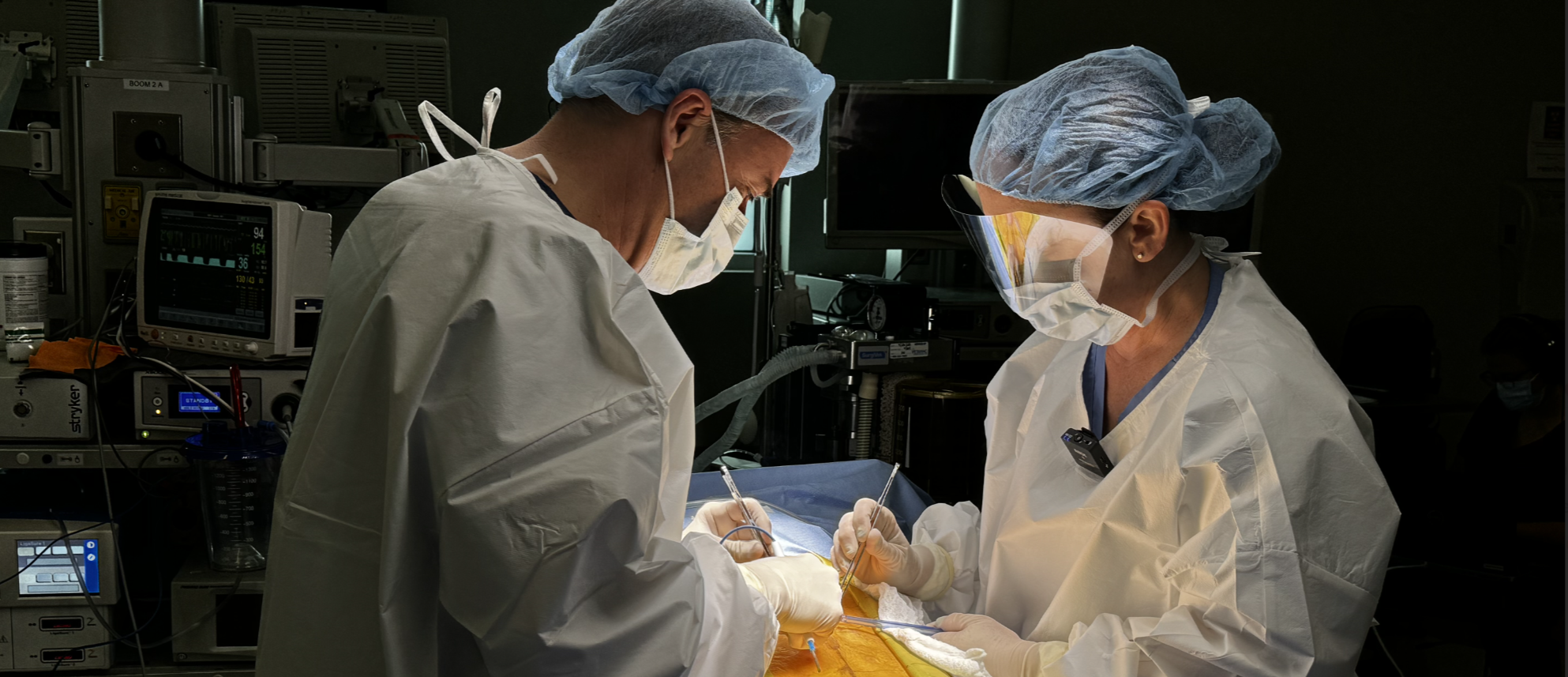
 Formative Usability Testing and Evaluation
Formative Usability Testing and Evaluation
Once prototypes are developed, formative evaluation is conducted to preliminarily assess the use-related risk mitigations as well as the degree to which the product is effective and easy to use. This is an iterative process, where feedback may be used to update user needs, user requirements, and the use-related risk analysis. This stage will often result in updates to the product user interface and labeling, as well as a determination whether and to what degree user training will be required.
There are two broad categories of formative evaluation, based on the degree to which representative users and use environment is involved. Read about Heuristic vs. Usability Studies here.
- Heuristic or Expert Reviews: “Quick and dirty” evaluations involving the assessment of a design by usability experts based on established Human Factors heuristics (rules of thumb).
- Formative Usability Testing: Usability testing with representative users by simulating product use in realistic scenarios. Formative testing can be performed either with a subset of tasks or the entire system, and with everything from early-stage prototypes to near validation-ready products. Read about Formative Studies here.
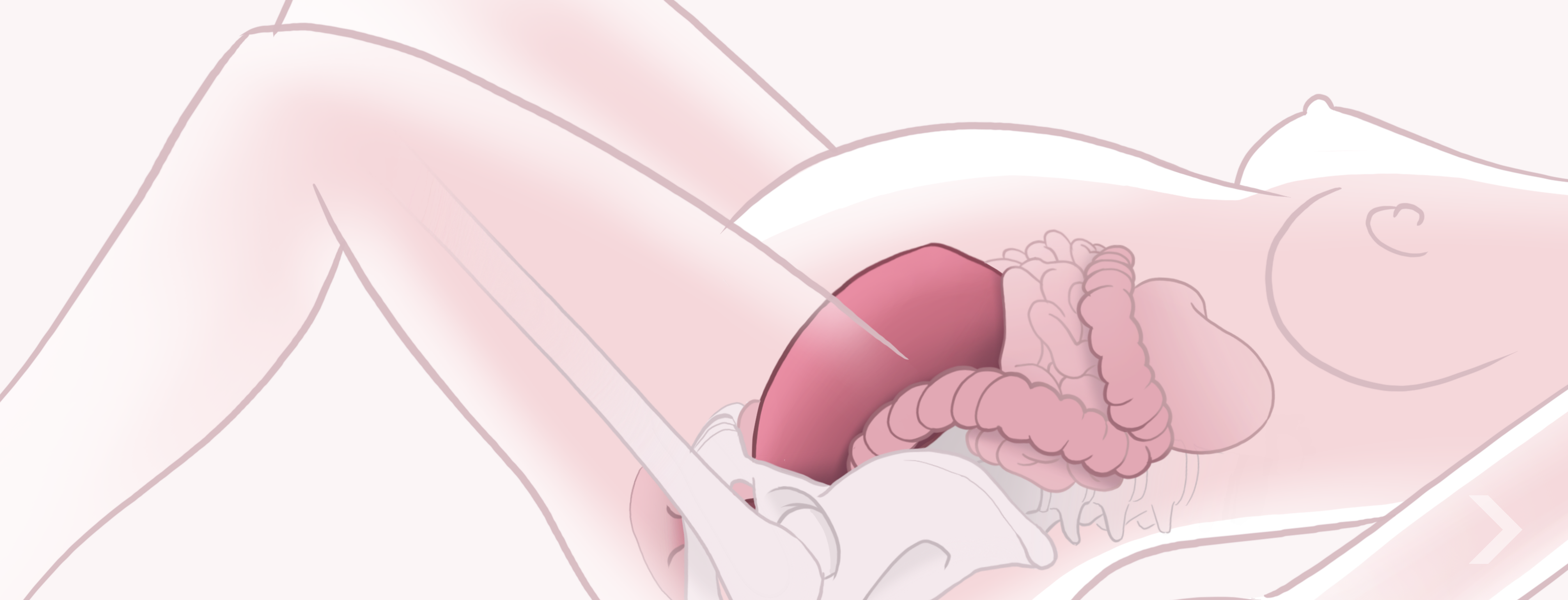
Summative Usability Validation
Once formative evaluations have demonstrated that the use-related risk mitigations are adequate and the product is sufficiently usable, usability validation testing (also referred to as summative evaluation) can be conducted.
The usability validation process involves formally validating that the intended users can safely and effectively use the product to perform all critical tasks, that all identified risks are adequately mitigated, and that the product performs as expected under (usually simulated) real-world conditions. Testing should simulate actual use conditions as closely as possible, with a sufficient sample size of representative users of all intended user groups.
For example, when Tandem Diabetes Care developed the world’s smallest insulin pump—Tandem Mobi—they partnered with Kaleidoscope to support formative and summative studies for FDA submission (learn more here).
Once usability validation is complete, a comprehensive Human Factors report is created as part of the regulatory submission for product approval.
Conclusion
The Human Factors engineering process for a medical product is complex and requires the expertise of experienced professionals to fully realize the potential of the product concept. But the result of a properly scoped and applied Human Factors process is a product that is safe, effective, and easy to use, making it more likely to result in a successful regulatory submission and ultimate success in the market.
For over 7 years, Kaleidoscope Innovation has been a trusted partner in Human Factors engineering, supporting industry leaders like Eli Lilly, Pfizer, and Baxter. Whether you're bringing a new device to market or refining an existing product, our team brings deep expertise and real-world insight to every stage of development. Let’s talk about how we can help you design safer, smarter, and more user-friendly medical products.
Back to Insights + News