Product Development Resources and Support
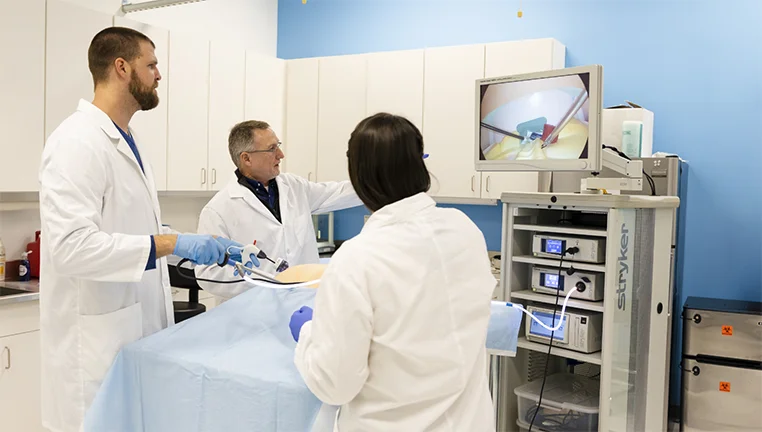
As part of Kaleidoscope Innovation, a world-leading design, product development, and consulting firm, we can offer clients more than just regulatory support, including end-to-end product development research and expertise as well as access to resources in emerging tech through our parent company, Infosys.
What’s more, our frequent close collaboration with Kaleidoscope’s multi-disciplinary departments—including engineering, human factors, and prototyping shop—gives us an immersive understanding of every phase of the product development process. It’s an invaluable level of insight and experience we bring to every project.
Get to Know Kaleidoscope Innovation
Collaborative Teams
We frequently collaborate closely
with these Kaleidoscope teams.
- Human Factors
- Engineering
- Machine + Electronics Shops
- Clinical Sciences
- Project Managment
- Quality Affairs